European scientists Scientists call upon EU for changes in legislation regarding new methods of plant genome editing
European scientists reach out to the European Parliament and the European Commission to change legislation regarding the use of modern methods of plant genome editing, which will free them to carry out research to benefit agriculture, the economy, and society as a whole.
Open Statement

Agriculture and food production must become more sustainable in a world facing an increasing, more affluent world population, climate change, and environmental degradation.

The recently published
Green Deal1 of the European Commission stated, within the context of the ‘Farm to Fork’
2 strategy, that the EU needs to develop innovative ways to reduce dependency on pesticides and fertilizers and reverse biodiversity loss while at the same time provide society with sufficient, nutritious, sustainable and affordable food. The strategy is in line with the importance of food and agriculture in achieving the United Nations’
Sustainable Development Goals (SDGs)
3.
Besides achieving these goals, we need to ensure a highly productive and sustainable recovery from the COVID-19 crisis, with an agriculture that is less dependent on imports from outside the EU.
However, setting the targets is not enough, we also need tools to help achieve these targets. All possible approaches, including innovative plant breeding technologies, are required to address these challenges and to achieve the ambitious goals of the Farm to Fork strategy. The most recent addition to the toolbox to develop new crop varieties is
precision breeding. This technology, also known as genome editing, allows scientists and breeders to develop desired crop varieties in a faster, relatively simple and much more directed way compared to previous breeding techniques. Precision breeding has far-reaching applications such as increasing the diversity of crops, the reduction of pesticides, the further development of healthy food, and many more.
A
greater diversity of crop species is not only desirable, but of central importance for both sustainable agriculture and healthy nutrition. The use of more varieties of crop species will increase the resilience to climate change. This crop diversity is especially important in a climate-smart approach because it contributes to pest and disease management, which has direct effect on yields and revenues.
4
Precision breeding can considerably
reduce the dependency on pesticides by improving resistance against diseases, as illustrated in recent literature with the development of e.g. mildew resistant wheat
5,6, fungal resistant grapevine
7, fungal resistant rice
8, broad-spectrum bacterial disease resistant tomato
9, grapefruit resistant to citrus canker
10, and rice resistant to bacterial blight
11-13.
Healthy food is the key to nutritious diets. Precision breeding accelerates the introduction of healthy traits into vegetables and fruits that we currently consume, e.g. high-fiber wheat
14, low-acrylamide potato
15, low gluten wheat
16, increased contents of beneficial secondary metabolites
14, reduced contents of allergens, and toxic heavy metals in cereals, legumes, and oilseeds
17-23.
However, the development of beneficial crop varieties in a faster and much more directed way is halted in Europe, while the rest of the world embraces the technology.
The ECJ ruling of 25 July 2018 in case C-528/16
24, which is widely interpreted to subject genome-edited plants to the general restrictive provisions of the European GMO legislation, in fact is preventing the use of this technology for crop improvement in Europe.
The
regulatory approach for genome-edited crops in Europe is completely out of line with the regulations existing in other continents across the world that have adopted more ‘fit for purpose’ regulations. The lack of regulatory harmonization worldwide poses challenges in global trade and in the seed sector and it hampers the innovation and scientific progress in Europe, which is very much needed for achieving Sustainable Development and Green Deal Goals.
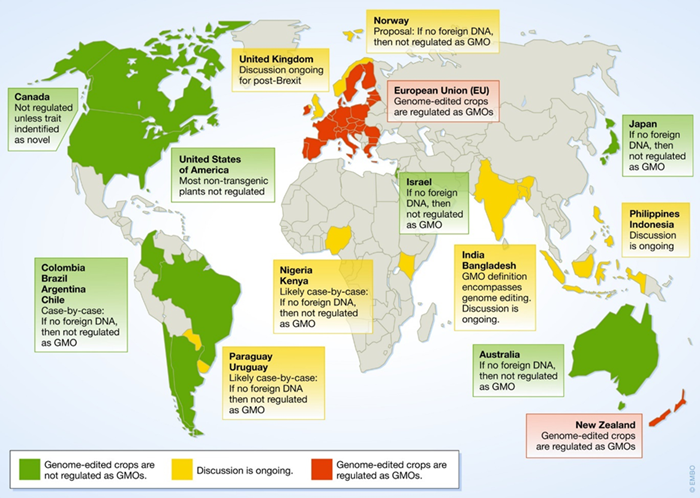
The figure below adopted from Schmidt
et al. provides a global overview of the regulatory approaches currently implemented or discussed in different countries for genome-edited crops (SDN-1 and SDN-2 applications)
25.
 The European Sustainable Agriculture through Genome Editing (EU-SAGE)26 network, with members from 132 European research institutes and associations, strongly recommends the following to the European Council, European Parliament and the European Commission:
The European Sustainable Agriculture through Genome Editing (EU-SAGE)26 network, with members from 132 European research institutes and associations, strongly recommends the following to the European Council, European Parliament and the European Commission:
European scientists advise
revising the existing GMO Directive to reflect current scientific knowledge and evidence on genome editing. Moreover, genome editing leading to the introduction of changes that can also occur naturally and which do not introduce foreign DNA should be exempted from the application of the GMO legislation (
cf. SDN-1 and SDN-2). In regulating genome editing, the legislator should consider the benefits of this technology, including the disadvantages from not adopting it.
Genome editing offers an increasing range of solutions for a more efficient selection of crops that are climate resilient, less dependent from fertilizers and pesticides, and help preserve natural resources. We recommend that the European Commission endorses this message for the benefit and welfare of all EU citizens.
While the legislation of many non-EU countries facilitates the use of genome editing, EU law distinguishes fundamentally between crops according to whether they are produced by genome editing or by traditional breeding methods.
There is an urgent need of harmonization of the regulatory framework worldwide.
Influential sectors of European society are not aware of the value of innovation in agriculture, including the one needed for preserving traditional varieties.
A narrative for European food production that includes the importance of innovative, more efficient approaches in the whole value chain is necessary.
References:
- https://eur-lex.europa.eu/resource.html?uri=cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0002.02/DOC_1&format=PDF
- https://eur-lex.europa.eu/resource.html?uri=cellar:ea0f9f73-9ab2-11ea-9d2d-01aa75ed71a1.0001.02/DOC_1&format=PDF
- https://www.un.org/sustainabledevelopment/sustainable-development-goals/
- http://www.fao.org/climate-smart-agriculture-sourcebook/production-resources/module-b1-crops/b1-overview/en
- Wang Y., X. Cheng, Q. Shan, Y. Zhang, J. Liu, et al., 2014 Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 32: 947–951. https://doi.org/10.1038/nbt.2969.
- Zhang Y, Bai Y, Wu G, Zou S, Chen Y, Gao C, Tang D., 2017 Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. Plant J.; 91:714–24. https://doi.org/10.1111/tpj.13599.
- Wang X, Tu M, Wang D, Liu J, Li Y, Li Z, et al., 2018 CRISPR/Cas9-mediated efficient targeted mutagenesis in grape in the first generation. Plant Biotechnol J., 16:844–55. https://doi.org/10.1111/pbi.12832.
- Wang F, Wang C, Liu P, Lei C, Hao W, Gao Y, et al., 2016 Enhanced rice blast resistance by CRISPR/Cas9-targeted mutagenesis of the ERF transcription factor gene OsERF922. PLoS ONE. 11:e0154027. https://doi.org/10.1371/journal.pone.0154027.
- de Toledo Thomazella DP, Brail Q, Dahlbeck D, Staskawicz BJ., 2016 CRISPR–Cas9 mediated mutagenesis of a DMR6 ortholog in tomato confers broad-spectrum disease resistance. 1–23. https://doi.org/10.1101/064824.
- Jia H, Zhang Y, Orbović V, Xu J, White FF, Jones JB, Wang N., 2017 Genome editing of the disease susceptibility gene CsLOB1 in citrus confers resistance to citrus canker. Plant Biotechnol J., 15:817–23. https://doi.org/10.1111/pbi.12677.
- Zhou J, Peng Z, Long J, Sosso D, Liu B, Eom J-S, et al., 2015 Gene targeting by the TAL effector PthXo2 reveals cryptic resistance gene for bacterial blight of rice. Plant J.;82:632–43. https://doi.org/10.1111/tpj.12838.
- Blanvillain-Baufumé S, Reschke M, Solé M, Auguy F, Doucoure H, Szurek B, et al., 2017 Targeted promoter editing for rice resistance to Xanthomonas oryzae pv. oryzae reveals differential activities for SWEET14-inducing TAL effectors. Plant Biotechnol J., 15:306–17. https://doi.org/10.1111/pbi.12613.
- Xie C, Zhang G, Zhang Y, Song X, Guo H, Chen X, Fang R., 2017 SRWD1, a novel target gene of DELLA and WRKY proteins, participates in the development and immune response of rice (Oryza sativa L.). Sci Bull.;62:1639–48. https://doi.org/10.1016/j.scib.2017.12.002.
- https://fdc.nal.usda.gov/
- Clasen BM, Stoddard TJ, Luo S, Demorest ZL, Li J, Cedrone F, et al., 2016 Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol J., 14:169–76. https://doi.org/10.1111/pbi.12370.
- Sánchez-León S, Gil-Humanes J, Ozuna CV, Giménez MJ, Sousa C, Voytas DF, Barro F., 2017 Low-gluten, nontransgenic wheat engineered with CRISPR/Cas9. Plant Biotechnol J. https://doi.org/10.1111/pbi.12837.
- Haun W, Coffman A, Clasen BM, Demorest ZL, Lowy A, Ray E, et al., 2014 Improved soybean oil quality by targeted mutagenesis of the fatty acid desaturase 2 gene family. Plant Biotechnol J., 12:934–40. https://doi.org/10.1111/pbi.12201.
- Demorest ZL, Coffman A, Baltes NJ, Stoddard TJ, Clasen BM, Luo S, et al., 2016 Direct stacking of sequence-specific nuclease-induced mutations to produce high oleic and low linolenic soybean oil. BMC Plant Biol., 16:225. https://doi.org/10.1186/s12870-016-0906-1.
- Wen S, Liu H, Li X, Chen X, Hong Y, Li H, et al., 2018 TALEN-mediated targeted mutagenesis of fatty acid desaturase 2 (FAD2) in peanut (Arachis hypogaea L.) promotes the accumulation of oleic acid. Plant Mol Biol., 97:177–85. https://doi.org/10.1007/s11103-018-0731-z.
- Zhou X, Liao H, Chern M, Yin J, Chen Y, Wang J, et al., 2018 Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc Natl Acad Sci USA.; 115:3174–9. https://doi.org/10.1073/pnas.1705927115.
- Abe K, Araki E, Suzuki Y, Toki S, Saika H., 2018 Production of high oleic/low linoleic rice by genome editing. Plant Physiol Biochem. https://doi.org/10.1016/j.plaphy.04.033.
- Nieves-Cordones M, Mohamed S, Tanoi K, Kobayashi NI, Takagi K, Vernet A, et al., 2017 Production of low-Cs+ rice plants by inactivation of the K+ transporter OsHAK1 with the CRISPR–Cas system. Plant J., 92:43–56. https://doi.org/10.1111/tpj.13632.
- Tang X., L. G. Lowder, T. Zhang, A. A. Malzahn, X. Zheng, et al., 2017 A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat Plants 3: 17018.
- Judgment of the Court of Justice of 25 July 2018. Confédération Paysanne and Others v. Premier Ministre and Ministre de L'Agriculture, de l'Agroalimentaire et de la Forêt. Case C-528/16. ECLI:EU:C:2018:583. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1590471867015&uri=CELEX:62016CJ0528
- Schmidt S.M., Belisle M., Frommer W.B. (2020). The evolving landscape around genome editing in agriculture: Many countries have exempted or move to exempt forms of genome editing from GMO regulation of crop plants. EMBO Rep 2020, e50680
- https://www.eu-sage.eu/